Introduction: PET/CT is the standard-of-care for staging and therapy response assessment in lymphoma. Fluorodeoxyglucose (FDG)-avidity in extranodal marginal zone lymphoma (EMZL) remains controversial with some variability by extranodal location. The current Lugano classification recommends the use of CT scans only in staging and follow-up for EMZL. However, emerging data from small studies have challenged this suggestion. In this study we aimed to determine the ability of PET/CT to detect FDG-avid EMZL during staging work-up.
Methods: First, we retrospectively analyzed the University of Miami (UM) MZL database (period 2/2016-1/2023) searching for patients who underwent staging PET/CT. Images were reviewed by expert radiologists to ascertain avidity of tumor and not surrounding tissue, with specific attention to ocular and GI locations; if scans were not available (n=15), we retrieved data from reports (results presented in part at ICML2023). Next, we aimed to validate the initial UM results additionally analyzing data from imaging reports for patients prospectively enrolled in the Lymphoma Epidemiology of Outcomes (LEO) MZL cohort (period 7/2015-5/2020), excluding LEO patients from Miami to avoid overlap. Patients with high-grade transformation at diagnosis were excluded. For patients with >1 extranodal site, each location was counted independently. We considered FDG-avid disease if SUVmax was ≥2 as this value is commonly observed between mediastinal blood pool (BP) and liver background. To normalize data, we calculated ratios of lymphoma SUVmax versus both BP and liver background, as available. Patients without measurable disease were excluded from analysis.
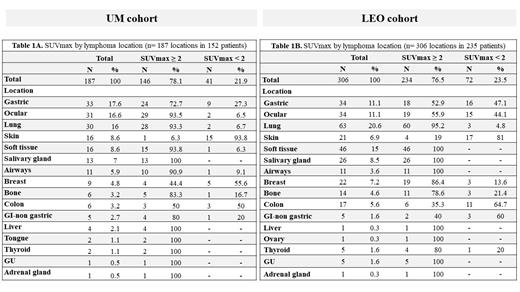
Results: Among 210 patients with EMZL in the UM cohort, 152 had staging PET/CT and were analyzed. Twenty-two patients demonstrated >1 extranodal site providing a total of 187 locations. The LEO cohort included 411 patients from 7 centers, with 235 having available imaging reports. Fifty-two patients in this cohort demonstrated >1 extranodal site providing a total of 306 locations. Disease distribution is depicted in Table 1. Among 187 EMZL locations in the UM cohort, the most common were gastric (n=33, 17.6%), ocular (n=31, 16.6%), lung (n=30, 16%), skin and soft tissue (n=16, 8.6%, each). Similarly, lung (n=63; 20.6%), soft tissue (n=46; 15%), gastric and ocular (n=34 each; 11%) were common locations in the LEO cohort. EMZL demonstrated FDG-avidity (SUVmax ≥2) across all locations with identical values in both cohorts (UM= 78.1% & LEO= 76.5%). FDG-avidity by location is shown in Table 1A & B with salivary gland (100% & 100%), lung (93.3% & 95.2%), soft tissue (93.8% & 100%), and airways (90.9% & 100%) demonstrating high avidity in both cohorts. Contrarily, skin was commonly non-FDG avid (93.8% & 81%). The median size of FDG-avid lesions was 2.4cm (range, 0.7-17.4) in UM and 2.9cm (range, 0.1-12) in LEO cohorts. In patients with >1 extranodal site FDG-avidity was nearly universal across sites (UM=93% & LEO=100%). We observed a significant correlation between lymphoma size and FDG-avidity for UM (r=0.33; P=0.001), and for LEO (r=0.55; P<0.0001). In patients with background FDG-avidity data in the UM cohort (n=150), a BP index and liver index ≥1 detected lymphoma in 79.3% & 71.5% cases, respectively. We were unable to calculate indexes in LEO cohort since only limited data on BP (n=26) and liver (n=19) background was available in imaging reports.
Conclusions: This study represents the largest analysis evaluating accuracy of PET/CT to determine initial tumor burden in 387 patients with EMZL. We demonstrate that EMZL is largely an FDG-avid disease across two cohorts, and PET/CT should be included in the staging of these patients while acknowledging possible limited sensitivity in GI locations due to physiologic uptake. Furthermore, we observed that patients with multiple extranodal locations demonstrate uniform FDG-avidity across all sites except for skin involvement. Based on our results we recommend the incorporation of PET/CT into standard staging of EMZL in planned revised Lugano classification guidelines. In those demonstrating initial FDG-avidity, a lower SUVmax threshold of SUVmax ≥2, comparison to mediastinal BP rather than to liver background activity and evaluating for resolution of focal FDG-avidity should be considered to assess treatment response in routine care and clinical trials.
Disclosures
Alderuccio:Genmab: Research Funding; Abbvie: Consultancy; Genentech: Consultancy; ADC Therapeutics: Consultancy, Research Funding. Koff:BeiGene: Consultancy; Viracta Therapeutics: Research Funding. Habermann:BMS: Research Funding; Genentech: Research Funding; sorrento: Research Funding. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; Regeneron: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; AbbVie: Honoraria; ADC Therapeutics: Honoraria. Cohen:Lam Therapeutics: Research Funding; BMS/Celgene: Research Funding; BioInvent: Research Funding; Novartis: Research Funding; Takeda,: Research Funding; Genentech: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Cerhan:NanoString: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding; Protagonist: Other: Safety Monitoring Committee; Genentech: Research Funding. Flowers:TG Therapeutics: Research Funding; V Foundation: Research Funding; Kite: Research Funding; Amgen: Research Funding; Pfizer: Research Funding; Takeda: Research Funding; 4D: Research Funding; Genentech Roche: Consultancy, Research Funding; National Cancer Institute: Research Funding; Ziopharm: Research Funding; Jannsen Pharmaceuticals: Research Funding; Acerta: Research Funding; Xencor: Research Funding; Allogene: Research Funding; Adaptimmune: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding; Beigene: Consultancy; Celgene: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Burroghs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Guardant: Research Funding; Cellectis: Research Funding; Spectrum: Consultancy; Genmab: Consultancy; Sanofi: Research Funding; Pharmacyclics: Research Funding; Novartis: Research Funding; Nektar: Research Funding; Gilead: Consultancy, Research Funding; Karyopharm: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Iovance: Research Funding; SeaGen: Consultancy; Pharmacyclics Jansen: Consultancy; Morphosys: Research Funding; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding. Lossos:LRF: Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria; NCI: Research Funding; University of Miami: Current Employment; NCI: Research Funding; BeiGene: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal